November 21, 2024 Chemtrails, Global Warming, Graphene, Scientific alternative studies
There are only two possible outcomes.
If the result confirms the hypothesis, then you have just made a measurement.
If the result contradicts the hypothesis, then you have made a discovery.
Chemical clouds from evaporation/volatilization/levitation of graphene-containing solutions and their ionization
Preface
One of the most complex phenomena to analyze, both because of its secrecy to date and the lack of freely available scientific documentation, is the issue of “chemtrails”.
In a previous article, I analyzed the article by (Herndon, Hoisington and Whiteside, 2020), which highlighted the existence of chemtrail clouds by direct spraying, whose emissions do not match the radiometric spectrum of water vapor.
The existence of “aerosol injections into the troposphere and stratosphere” based on silica aerogels has also been clarified, with the possibility of using others based on graphene (Vukajlovic, J.; Wang, J.; Forbes, I.; Šiller, L. 2021), as mentioned in the previous article on solar geoengineering.
Thus, it is clear that regardless of the ultimate motive or purpose of these actions, the spraying of the skies is now an indisputable fact, as evidenced by studies of aerosol samples (Pöschl, U. 2005 | Shiraiwa, M.; Sosedova, Y.; Rouvière, A.; Yang, H.; Zhang, Y.; Abbatt, JP; Pöschl, U. 2011).
This paper explores the possibility that chemical clouds observed in the sky may not be exclusively due to chemtrails or aircraft spraying.
The phenomenon of chemical clouds may be much more complex than it appears at first glance.
In fact, there is a very high probability that chemical clouds are produced by the evaporation effect of water, fertilizers, pesticides, food additives, and graphene.
In order to thoroughly analyze this phenomenon, it is necessary to study the following references: the accelerated evaporation of water on graphene oxide, the evaporation of pesticides in the agricultural environment, the levitation of graphene and the effects of its ionization.
Water evaporation studies with graphene
Among the applications of graphene are those related to water, either for its filtration or for its decontamination (Sun, XF; Qin, J.; Xia, PF; Guo, BB; Yang, CM; Song, C.; Wang, SG 2015 | Xu, C .; Cui, A .; Xu, Y. ; Fu, X. 2013 | Fathizadeh, M .; Xu, WL ; Zhou, F .; Yoon, Y .; Yu, M. 2017), to control its evaporation.
In this section, we will analyze the latter application in particular.
In particular, the work of Wan and Shi (2017), which aims to discover the best way to achieve the maximum possible evaporation of water in contact with graphene, deserves attention.
The researchers state that “the evaporation of tiny and even nanoscale volumes of water on solid surfaces is of paramount importance in a wide range of biological and industrial processes, such as transpiration, medical diagnostics, chip fabrication, spray cooling, and inkjet printing“.
Among these uses and applications, spraying/fogging finds wide use in agriculture, in irrigation through “micro-aspersions” that promote the proper moisture content and temperature of crops.
According to Wan and Shi (2017), it was found that “nanoscale water evaporation on patterned hydrophobic-hydrophilic surfaces is unexpectedly faster than on any surface with uniform wettability, and this improvement is related to the size of the patterned domain“.
Graphene and graphene oxide are suitable materials to serve as catalysts in this evaporation process due to their dispersibility and adsorption capacity.
In addition, it was concluded that “evaporation is remarkable in the unoxidized regions” of the graphene film used in the experiment.
On the other hand, it has also been found that “reducing the thickness of the water increases the influence of the solid surface on the outermost water molecules and prolongs the lifetime of hydrogen bonds in these molecules, making it more difficult for the outermost water molecules to evaporate“.
This means that water evaporation varies depending on the integrity of the molecular structure of graphene.
This opens up the possibility of controlling or regulating the evaporation process.
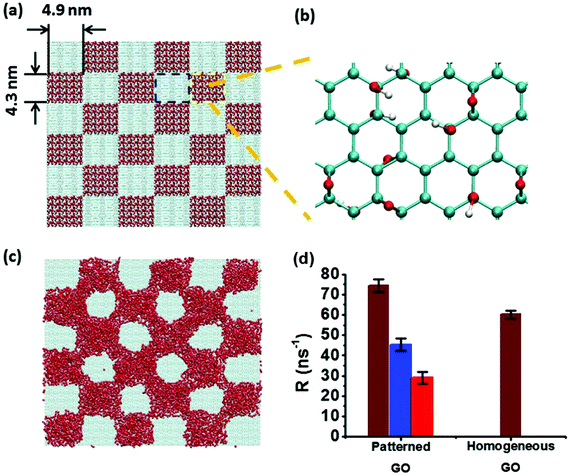
The work of (Huang, Y.; Lu, J.; Meng, S., 2018) supports these findings, stating that “a graphene coating controls water evaporation by suppressing the evaporation rate on hydrophilic surfaces and accelerating evaporation on hydrophobic surfaces“.
In addition, the authors note that “graphene is transparent to evaporation.
When a hydrophilic surface is covered with graphene, the contact line of the water droplet is drastically shortened or lengthened due to the adjustment of wetting angles.
This leads to changes in the evaporation rate“.
These findings clarify that water can evaporate depending on the molecular structure of graphene and its degree of oxidation, an observation also confirmed by (Tong, WL; Ong, WJ; Chai, SP; Tan, MK; Hung, YM 2015).
These facts suggest that graphene could also be found together with water vapor depending on its weight and molecular structure, which will be confirmed in the next exposition of facts.
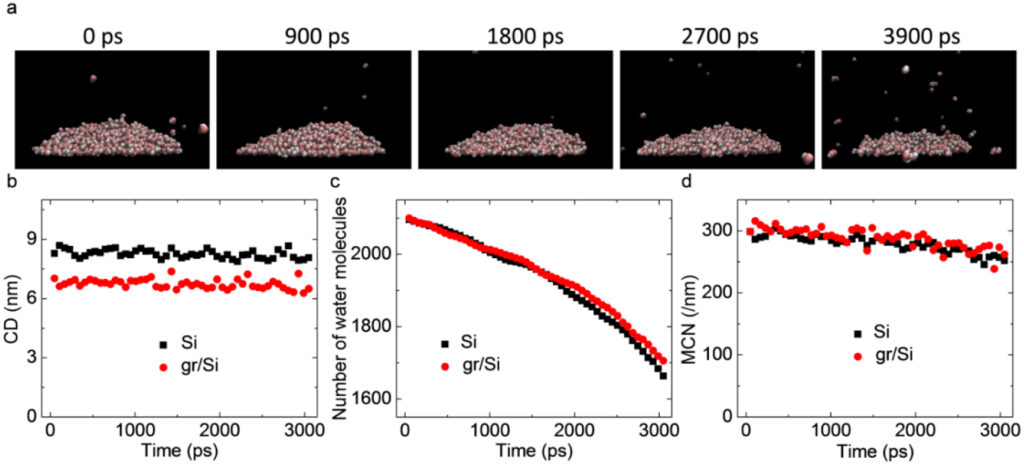
Evaporation of graphene is possible under temperature and pressure conditions similar to those observed in the presence of “COVID”, i.e. in the presence of both ionizing and non-ionizing electromagnetic radiation absorbed by graphene oxide.
This is demonstrated by the research of (Grinchuk, P.S.; Fisenko, E.I.; Fisenko, S.P.; Danilova-Tretiak, S.M., 2020), in which the authors analyze the rate of isothermal evaporation of liquid aerosols and the survival of so-called “COVID” in such conditions.
The observations of the researchers are very unique : “The observed effect of the decrease in the concentration of viable viruses in an aqueous sample during evaporation from a solid substrate in the experiments confirms our hypothesis about the existence of the physical mechanism.
There is at least a qualitative analogy confirmed by several experimental data.
It has been shown experimentally that a graphene oxide sheet undergoes significant deformation in a one-micron drop of water during evaporation.
Graphene is a very strong material, with a Young’s modulus of up to 1 TPa (Terapascal)“.
Researchers have unconsciously found evidence that the so-called “COVID” has the same mechanical properties as graphene oxide (Wang, WN; Jiang, Y.; Biswas, P. 2012 | Frank, IW; Tanenbaum, DM; van der Zande, AM; McEuen, PL 2007), qualitatively consistent with its evaporation rate and morphology.
This could be because the researchers most likely observed a form of graphene oxide that had the same appearance as the so-called (remember, no one has ever proven its existence either sequentially or in isolation) “COVID,” as will be explained in the next section.
Studies on evaporation, volatilization and levitation of pesticides and fertilizers
Considering that graphene oxide can evaporate when in aqueous or liquid solutions, as just explained, it would not be surprising that its intensive use in agriculture in the form of fertilizers and pesticides could be partly due to its evaporation, with the obvious consequence of chemical cloud formation.
In this regard, the contribution of (Peterson, EM; Green, FB; Smith, PN, 2020) is crucial in clarifying whether chemical cloud formation from fertilizers, pesticides, and veterinary drugs is possible.
In their abstract, they note the recent discovery of “airborne transport of veterinary pharmaceuticals from industrial livestock feeding operations through particulate matter“.
This confirms that one way to treat livestock is through aerosol clouds containing the drugs needed to treat them (McEachran, AD; Blackwell, BR; Hanson, JD; Wooten, KJ; Mayer, GD; Cox, SB; Smith, PN 2015).
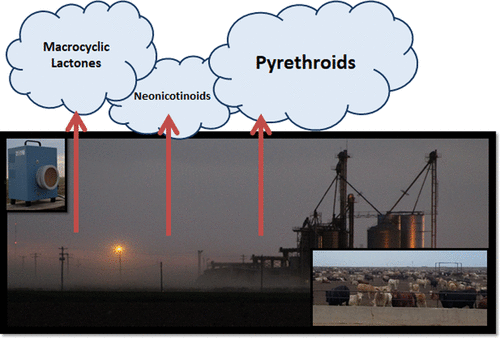
However, the purpose of his research is to determine “the extent to which insecticides are also transported to the environment from total suspended particles emitted from beef cattle feedlots.
Of 16 different pesticides quantified in particulate samples collected from beef cattle at feedlots, permethrin was detected with a higher frequency of more than 67 percent and an average concentration of 1211.7 ± 781.0 (SE) ng/m3“.
This statement shows that pesticides and crop protection products used on farms were found in chemical clouds in very high proportions.
Added to this statement is an even more important one: “Imidacloprid was detected at a mean concentration of 62.8 ± 38.2 (SE) ng/m3, corresponding to published concentrations for dust resulting from treated seeding activities.
This result is very important because imidacloprid (C₉H₁₀ClN₅O₂) is a neonicotinoid, a nicotine-based neuroactive insecticide that is applied foliarly or radically through irrigation water.
Interestingly, there are patents for graphene oxide with “imidacloprid”, but under the name “paichongding”.
This could be due to the implementation of Directive 98/8/EC of the European Parliament and of the Council of February 16, 1998 on the marketing of biocidal products, which prohibits the use of imidacloprid, abamectin, avermectin and other chemical compounds that are clearly harmful to health, except in closed greenhouses (due to their volatility and evaporation).
According to patent CN107581193A (Zhou Chengyan, Li Zhong, Wu Chengwei, Xu Xiaoyong, Xiong Yanling, Shao Xusheng, Wu Jing, Lu Jing, Wu Yanhui, Liang Ying, Zhou Qiwen, 2016), “Paichongding” is an insecticide composed of chloropyridine pyridine and “hexahydro-imidazoles” or “imidazole”, which is precisely one of the components of “imidacloprid”, according to the International Labor Organization (ILO) list of hazardous substances.
Appendix 1 of this entry includes some patents related to graphene oxide with Avermectin and Paichongding.
Returning to the analysis of (Peterson, EM; Green, FB; Smith, PN 2020), we also find that “over the past 50 years, many feed companies have been established in areas that receive relatively little precipitation, similar to the High Plains.
These regions (plains of the United States, Mexico, South America, and northern Australia) are often subject to drought, which contributes to the formation of particulate matter and the release of pesticides from feedlots, resulting in the release of insecticides in the form of PM (microparticles) into the local environment.
This situation is likely to occur in food courts around the world, regardless of climatic conditions“.
The work of (Ul-Islam, Nisar, Kmail, and Umar, 2018) shows that graphene is intensively used in agricultural soils to fix all types of fertilizers (urea and nitrogen), which experience a volatility of about 40 percent after 24 hours.
Other works share the analysis of the problem (Yuan, W.; Shen, Y.; Ma, F.; Du, C. 2018) and show that the losses are due to three factors : a) volatilization in the form of ammonia, which contributes to the greenhouse effect ; b) leaching in the form of nitrates, which causes eutrophication of water bodies ; c) runoff.
Therefore, researchers have considered creating a graphene oxide compound with polyacrylate polymer to avoid these problems.
It is well known that the adsorption capacity of graphene oxide allows the controlled release of fertilizers.
However, it is also known that ultraviolet radiation breaks down the molecular structure of graphene oxide, creating quantum dots that cause the release of adsorbed chemicals, promoting their evaporation and volatilization, and the formation of clouds of suspended particles by levitation.
In this investigation, it was concluded that “the aerogel prepared for the experiment could be instantly heated by a halogen lamp due to its high light absorption capacity and low heat capacity.
When heated, the aerogel is levitated by the buoyancy of the surrounding air, and the levitation behavior could be controlled by the on/off cycle of the light source.
Research on the levitation of CNT aerogel (carbon nanotubes, graphene oxide in tubular-cylindrical form) using sunlight is ongoing“.
CNT aerogel was tested with densities ranging from 0.25 to 1 mg/cm³, and instantaneous levitation was achieved with a heating rate of 17 °C/s.
This effect was observed by Zhang, T.; Chang, H.; Wu, Y.; Xiao, P.; Yi, N.; Lu, Y.; Chen, Y. (2015) in their work on macroscopic propulsion with direct light on bulk graphene.
They found that “macrographene-based objects can be directly propelled by watt-level lasers, and even by sunlight, down to the sub-meter scale….” ….. The propulsion could be further enhanced by increasing the intensity of the light and/or improving the illuminated area“.
Effects of ionization on graphene
The effects of ionization on graphene are complex because it causes desorption of ions and generation of ionized species (free radicals), with an opposite effect to that of adsorption.
According to Kim, Na, Kwack, Ryoo, Lee, Hong and Min (2011), when an ionizing laser is applied to graphene oxide, it transforms into reduced graphene oxide rGO and then forms multi-walled carbon/graphene nanotubes known as MWCNTs.
This GO-reducing effect was also observed in the study by (Cutroneo, M.; Havranek, V.; Mackova, A.; Malinsky, P.; Torrisi, L.; Lorincik, J.; Stammers, J., 2019), who state, “an ionic microring is an effective way to deoxygenate graphite oxide sheets and produce a reduced graphene oxide to improve the relative carbon content (C/O, carbon/oxygen ratio) and increase electrical conductivity”.
This study provides important evidence that reduced graphene oxide increases electrical conductivity, so its presence in chemical or hybrid clouds with water vapor and other pollutants can increase the electrical activity of the atmosphere.
To complete the picture, it is important to mention the work of (Wang, Z., Yu, C., Huang, H., Guo, W., Yu, J. and Qiu, J., 2021), which provides a holistic view of the effects of microwaves on carbon chemistry, especially at the nanoscale.
The authors state that “microwaves are electromagnetic waves characterized by sinusoidal variation of electric and magnetic fields.
The frequency of microwaves is between 300 MHz and 300 GHz, with the 2.45 GHz frequency being more widely used“.
The heat conducted by electromagnetic radiation causes polarization and excitation of carbon-based materials, raising their temperature and causing the effects of desorption, exfoliation, reduction and doping.
The most significant effect, however, is “the strong interaction between the nearly free-moving electrons and the electric field, which increases the kinetic energy of these electrons by allowing them to rapidly jump out of the conjugate region on the carbon surface, resulting in ionization of the gaseous species and a noticeable emission of light in a limited time and space.
This phenomenon is perceived as an arc or plasma discharge.
The intense generation of such ionized species/plasmas may have great potential for the microwave reactions involved, due to the characteristics of microscale size and uniquely high energy density“.
Final Thoughts
There is now ample evidence that graphene, when combined with other components (found in fertilizers and pesticides), if it has sufficient density, a high degree of porosity, and is in a liquid solution susceptible to evaporation, is capable of forming chemical clouds.
It can also be carried by hot air currents and form chemical dust clouds with other materials.
Based on current scientific knowledge, it is highly likely that graphene is present in clouds as a result of a heating and evaporation process, especially in summer and dry climates.
Considering that there are chemical clouds in which there may be a concentration of graphene or graphene oxide that has not yet been experimentally quantified (in the absence of further studies), it is highly likely that they also have an impact on the increase in electromagnetic activity.
All this, together with the effect of electromagnetic waves (microwaves), the multiplier effect and the electromagnetic absorption of graphene oxide, causes the ionization of chemical clouds, generating a desorption effect that leads to the precipitation of water, fertilizers, pesticides, herbicides or nucleated chemical compounds in their aerosol phase in the air.
It is also suggested that the ionization of graphene may cause the release of free radicals and ionized species, which could be the source of the unusually high radiation levels.
Obviously, the ionizing radiation pulses appear to be outside the natural pattern of the phenomenon.
However, it is possible that electromagnetic pulses from weather, military, and air traffic surveillance radars cause a rebound effect on magnetized graphene oxide (and presumably Fe₃O₄ magnetite) particles, producing an ionizing radiation pulse.
Therefore, it is essential to observe and study the atmospheric aerosols and to localize the radiation pulses to verify their origin and exclude other hypotheses.
Bibliography
1.CN107581193A. (2016). A kind of Pesticidal combination containing paichongding and pymetrozine based on carrier. https://patents.google.com/patent/CN107581193A/en
2.Cutroneo, M.; Havranek, V.; Mackova, A.; Malinsky, P.; Torrisi, L.; Lorincik, J.; Balbetta, J. (2019). Localized deoxygenation of graphene oxide foil by ion microbeam writing. Vacuum, 163, pp. 10-14. https://doi.org/10.1016/j.vacuum.2019.01.055
3.Fathizadeh, M.; Xu, WL; Zhou, F.; Yoon, Y.; Yu, M. (2017). Graphene oxide: a novel 2‐dimensional material in membrane separation for water purification. Advanced Materials Interfaces, 4 (5), 1600918. https://doi.org/10.1002/admi.201600918
4.Frank, IW; Tanenbaum, DM; van der Zande, AM; McEuen, PL (2007). Mechanical properties of suspended graphene sheets. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena 25 (6), pp. 2558-2561. https://doi.org/10.1116/1.2789446
5.Grinchuk, PS; Fisenko, EI; Fisenko, SP; Danilova-Tretiak, SM (2020). Isothermal evaporation rate of deposited liquid aerosols and the SARS-CoV-2 coronavirus survival. arXiv preprint arXiv, 2004.10812. https://arxiv.org/pdf/2004.10812.pdf
6.Huang, Y.; Lu, J.; Meng, S. (2018). Transparency in graphene mediated evaporation. 2D Materials, 5 (4), 041001. https://doi.org/10.1088/2053-1583/aac9ff
7.Kim, YK; Na, HK; Kwack, SJ; Ryoo, SR; Lee, Y.; Hong, S.; Min, DH (2011). Synergistic Effect of Graphene Oxide/MWCNT Films in Laser Desorption/Ionization Mass Spectrometry of Small Molecules and Tissue Imaging, Acs Nano, 5 (6), pp. 4550-4561. https://doi.org/10.1021/nn200245v
8.McEachran, AD; Blackwell, BR; Hanson, JD; Wooten, KJ; Mayer, GD; Cox, SB; Smith, PN (2015). Antibiotics, bacteria, and antibiotic resistance genes: aerial transport from cattle feed yards via particulate matter. Environmental health perspectives 123 (4): pp. 337-343. https://doi.org/10.1289/ehp.1408555
9.Peterson, EM; Green, FB; Smith, PN (2020).Pesticides used on beef cattle feed yards are aerially transported into the environment via particulate matter. Environmental Science & Technology, 54 (20), pp. 13008-13015. https://doi.org/10.1021/acs.est.0c03603
10.Poschl, U. (2005). Atmospheric aerosols: composition, transformation, climate and health effects. Angewandte Chemie International Edition, 44 (46), pp. 7520-7540. https://doi.org/10.1002/anie.200501122
11.Shiraiwa, M.; Sosedova, Y.; Rouvière, A.; Yang, H.; Zhang, Y.; Abbatt, JP; Poschl, U. (2011). The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nature Chemistry, 3 (4), pp. 291-295. https://doi.org/10.1038/nchem.988
12.Sun, XF; Qin, J.; Xia, PF; Guo, BB; Yang, CM; Song, C.; Wang, SG (2015). Graphene oxide–silver nanoparticle membrane for biofouling control and water purification. Chemical Engineering Journal, 281, pp. 53-59. https://doi.org/10.1016/j.cej.2015.06.059
13.Tong, WL; Ong, WJ; Chai, SP; Tan, MK; Hung, YM (2015).Enhanced evaporation strength through fast water permeation in graphene-oxide deposition. Scientific Reports, 5 (1), pp. 1-13. https://doi.org/10.1038/srep11896
14.ul-Islam, S.; Nisar, S.; Kmail, A.; Umar, A. (2018). A review on immobilization techniques for fertilizer. International Journal of Chemical and Biochemical Sciences 14, pagg. 88-94. https://www.researchgate.net/profile/Shafaq-Nisar/publication/336135131_A_review_on_immobilization_techniques_for_fertilizer/links/5d91d1a892851c33e948a1c6/A-review-on-immobilization-techniques-for-fertilizer.
15.Vukajlovic, J.; Wang, J.; Forbes, I.; Ciller, L. (2021). Diamond-doped silica aerogel for solar geoengineering. Diamond and Related Materials, 108474. https://doi.org/10.1016/j.diamond.2021.108474
16.Wan, R.; Shi, G. (2017). Accelerated evaporation of water on graphene oxide. Física Química, 19 (13), pp. 8843-8847. https://doi.org/10.1039/C7CP00553A
17.Wang, WN.; Jiang, Y.; Biswas, P. (2012). Evaporation-induced crumpling of graphene oxide nanosheets in aerosolized droplets : confinement force relationship. The journal of physical chemistry letters 3 (21), pp. 3228-3233. https://doi.org/10.1021/jz3015869
18.Wang, Z., Yu, C., Huang, H., Guo, W., Yu, J., Qiu, J. (2021). Carbon-Enabled Microwave Chemistry : from Interaction Mechanisms to Nanomaterial Manufacturing. Nano Energy 106027. https://doi.org/10.1016/j.nanoen.2021.106027
19.Xu, C.; Cui, A.; Xu, Y.; Fu, X. (2013). Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon, 62, pp. 465-471. https://doi.org/10.1016/j.carbon.2013.06.035
20.Yanagi, R.; Takemoto, R.; Ono, K.; Ueno, T. (2021). Light-induced levitation of ultralight carbon aerogels via temperature control. Scientific Reports, 11 (1), pp. 1-8. https://doi.org/10.1038/s41598-021-91918-5
21.Yuan, W.; Shen, Y.; Ma, F.; Du, C. (2018). Application of Graphene-Oxide-Modified Polyacrylate Polymer for Controlled-Release Coated Urea. Coatings, 8 (2), 64. https://doi.org/10.3390/coatings8020064
22.Zhang, T.; Chang, H.; Wu, Y.; Xiao, P.; Yi, N.; Lu, Y.; Chen, Y. (2015). Macroscopic and direct light propulsion of bulk graphene material. Nature Photonicsa, 9 (7), pp. 471-476. https://doi.org/10.1038/nphoton.2015.105
Appendix
1.CN107439568A. A kind of composition pesticide containing Avermectin B2. https://patents.google.com/patent/CN107439568A/en
2.CN107711861A. A kind of go out controls the attractant and preparation method of whole nest Soil termites. https://patents.google.com/patent/CN107711861A/en
3.CN109497048A. A kind of trunk injection liquor of containing graphene nano material. https://patents.google.com/patent/CN109497048A/en
4.CN109704321A. A kind of nano graphene oxide and its preparation and application. https://patents.google.com/patent/CN109704321A/en
5.CN111727964A. Avermectin nano slow-release pesticide preparation and preparation method thereof. https://patents.google.com/patent/CN111727964A/en