November 14, 2024 Chemtrails, Global Warming, Graphene, Scientific alternative studies
Not surprisingly, then, independent analyses have led to the discovery of graphene oxide nanosheets in rainwater.
Graphene oxide and ice nucleation in the atmosphere
Studio di riferimento
Joghataei, M.; Ostovari, F.; Atabakhsh, S.; Tobeiha, N. (2020). Heterogeneous ice nucleation by Graphene nanoparticles. Scientific reports, 10 (1), pp. 1-9. https://doi.org/10.1038/s41598-020-66714-2
Facts analyzed
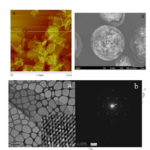
This study investigated the potential of “graphene oxide nanoparticles“, called “GGONs“, as a basis for ice nucleation, or the formation of ice crystals.
The researchers observed that GGON nanoparticles, ranging in size from 160 to 180 nanometers, promoted the formation of ice crystals at temperatures ranging from -20 to -10 degrees Celsius.
The experiment was conducted in a fog chamber that simulates the conditions of aerosol clouds in the Earth’s atmosphere.
As reported in the article, “more than 50 percent of the precipitation on Earth originates in the ice phase, and particles that can act as ice nuclei (IN) are essential for cloud and precipitation microphysics.
Nowadays, humans are trying to modify clouds to increase their water resources, and in this context, artificial aerosols that contribute to cloud microphysics in cloud seeding are desired.
Therefore, aerosol-cloud interactions and their effects on weather, climate and climate change are among the major environmental problems“.
This again confirms that cloud seeding and cloud modification by aerosol injection are now common procedures to maximize precipitation and consequently modify the climate.
The process of ice crystal formation in the atmosphere is explained in detail : “Heterogeneous ice nucleation generally requires an insoluble AP (aerosol particle) to serve as the IN (ice nucleus), which initiates the ice phase by direct water vapor deposition, freezing by aqueous medium, and by contact, immersion, or condensation of specific PAs“.
In this case, “GGON” graphene oxide nanoparticles act as the AP aerosol particles.
The researchers point out that graphene oxide in the form of GGON has ideal characteristics for ice nucleation due to its two-dimensional structures and thermal and mechanical properties, as demonstrated in their experiment, with better results than other materials commonly used for ice cloud seeding, such as silver iodide and kaolinite powder.
According to the authors, “although graphene G is hydrophobic, graphene oxide GO is instead hydrophilic, and the presence of these two properties side by side favors ice nucleation“.
Another factor that favors ice nucleation is the absence or low amount of organic carbon in the material, which favors crystallization.
Other studies confirm the above results.
For example, (Xue, H.; Lu, Y.; Geng, H.; Dong, B.; Wu, S.; Fan, Q.; Wang, J. 2019) state that the density of “hydroxyl groups,” also known as HOPGs (highly oriented pyrolytic graphites), including graphene, increases ice nucleation activity.
In addition, the authors make other very relevant statements about the importance of ice formation in the atmosphere : “Ice crystal formation is crucial in atmospheric science.
For example, ice crystals provide a medium for the exchange of atmospheric molecules and particles within the ecosystem.
Furthermore, ice crystals also act as reactive hosts that influence the concentration of ozone in the stratosphere“.
On the other hand, “less oxidized graphene sheets can nuclearize ice more efficiently“, a property also confirmed by (Hausler, T.; Gebhardt, P.; Iglesias, D.; Rameshan, C.; Marchesan, S.; Eder, D.; Grothe, H., 2018) and (Whale, T.F.; Rosillo-López, M.; Murray, B.J.; Salzmann, C.G. 2015).
Final thoughts
Ice nucleation in the atmosphere is a key aspect of climate research, with the goal of controlling precipitation, temperatures and, consequently, increasingly needed water resources.
Silver iodide and kaolinite are giving way to the use of 2D nanomaterials such as graphene oxide, which are more productive in forming ice nanocrystals.
Tests performed by (Joghataei, M.; Ostovari, F.; Atabakhsh, S.; Tobeiha, N. 2020) in a fog chamber simulating aerosol conditions in the atmosphere at -20 °C are similar to those found in the troposphere at about 7-8 km altitude.
At the altitude at which commercial aircraft typically fly, about 10 km (at the boundary of the troposphere with the tropopause), the temperature can reach -60 °C.
These details are important because “ice crystals formed in the upper troposphere and lower stratosphere (upper troposphere UT/lower stratosphere LS) can settle, reducing moisture and causing dehydration of the upper troposphere UT.
This affects the distribution of water vapor and thus the radiative budget, since water vapor is the strongest greenhouse gas.
Ice particles in the tropopause control the transport of water in the lower LS stratosphere, which in turn affects the chemical composition of the stratosphere.
Ice crystal surfaces can act as heterogeneous surfaces for ozone depletion reactions and as sinks for nitric acid (HNO3)“.
In other words, if it is true that GO graphene oxide is injected at an altitude between 7 and 10 km (upper troposphere and tropopause), i.e. the same range where commercial airplanes usually fly, it not only generates ice nucleation, but also causes ozone depletion and dehydration of the upper troposphere.
To these serious problems we must add the known toxicity and adverse effects of graphene on the body.
While it is true that graphene oxide sprays are already being carried out in the troposphere, they could pursue several objectives : a) cloud formation and seeding ; b) precipitation and water resources capture ; c) climate modification/geoengineering.
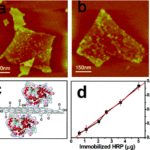
Indeed, in their study (Liang, H. ; Möhler, O. ; Griffiths, S. ; Zou, L. 2019), the authors conclude : “By observing ice nucleation in PrGO-SN (porous graphene oxide and silica dioxide) compounds under E-SEM (scanning electron microscope), we found that the porous PrGO-SN compound showed the initiation of ice nucleation at a higher temperature (-8 °C), as well as rapid and continuous ice crystal growth.
These results contribute to a better understanding of the factors influencing the process of heterogeneous ice nucleation and offer new perspectives for the design and fabrication of functional materials for porous ice nucleation, with possible practical applications such as rain enhancement and cloud formation by cloud seeding“.
This statement clearly shows the intention to improve the precipitation by the graphene oxide GO ice nucleation method and even to measure it with specially prepared drones (Bieber, P.; Seifried, TM; Burkart, J.; Gratzl, J.; Kasper-Giebl, A.; Schmale, DG; Grothe, H. 2020).
Therefore, it is not surprising that independent analyses were performed that led to the discovery of graphene oxide nanosheets in rainwater.
Bibliography
1.Bieber, P.; Seifried, TM; Burkart, J.; Gratzl, J.; Kasper-Giebl, A.; Schmale, DG; Grothe, H. (2020). A drone-based bioaerosol sampling system to monitor ice nucleation particles in the lower atmosphere. Remote Sensing, 12 (3), 552. https://doi.org/10.3390/rs12030552
2.Häusler, T.; Gebhardt, P.; Iglesias, D.; Rameshan, C.; Marchesano, S.; Eder, D.; Grothe, H. (2018). Ice nucleation activity of graphene and graphene oxides. The Journal of Physical Chemistry. 122 (15), pp. 8182-8190. https://doi.org/10.1021/acs.jpcc.7b10675
3.Knopf, DA; Alpert, PA; Wang, B. (2018). The role of organic aerosol in atmospheric ice nucleation: a review. ACS Earth and Space Chemistry, 2 (3), pp. 168-202. https://doi.org/10.1021/acsearthspacechem.7b00120
4.Li, JM; Chang, PH; Li, L.; Teo, CJ; Koo, a.C.; Duan, H.; Mai, VC (2018). Application of Graphene Oxide in Jet A-1 in Air to Enhance Combustion Process. En 2018 AIAA Aerospace Sciences Meeting. pp. 133. https://arc.aiaa.org/doi/abs/10.2514/6.2018-0133
5.Liang, H.; Möhler, O.; Griffiths, S.; Zou, L. (2019). Enhanced Ice Nucleation and Growth by Porous Composite of RGO and Hydrophilic Silica Nanoparticles. The Journal of Physical Chemistry C, 124 (1), pp. 677-685.https://doi.org/10.1021/acs.jpcc.9b09749
6.Whale, TF; Rosillo-López, M.; Murray, BJ; Salzmann, CG (2015). Ice Nucleation Properties of Oxidized Carbon Nanomaterials. https://doi.org/10.1021/acs.jpclett.5b01096
7.Xue, H.; Lu, Y.; Geng, H.; Dong, B.; Wu, S.; Fan, Q.; Wang, J. (2019). Hydroxyl Groups on the Graphene Surfaces Facilitate Ice Nucleation. The journal of physical chemistry letters, 10 (10), pp. 2458-2462. https://doi.org/10.1021/acs.jpclett.9b01033
8.Zabarnick, S.; DeWitt, MJ; Striebich, RC; Gunasekera, TS; Ervin, JS; Brioni, AM; Harruff Miller, BA (2016). Catalytic routes for the conversion of lignocellulosic biomass to aviation fuel range hydrocarbons. Renewable and Sustainable Energy Reviews, 120, 109612. https://doi.org/10.1016/j.rser.2019.109612