December 29, 2024 Global Warming, Graphene, Scientific alternative studies
To reduce greenhouse gases, graphene oxide can be used to adsorb CO2 from the atmosphere.
Graphene Oxide Can Both Adsorb and Absorb CO2
Reference Study
Rodríguez-Garcia, S.; Santiago, R.; López-Díaz, D.; Merchan, MD; Velázquez, MM; Fierro, JLG; Palomar, J. (2019). Role of the Structure of Graphene Oxide Sheets on the CO2 Adsorption Properties of Nanocomposites Based on Graphene Oxide and Polyaniline or Fe3O4 – Nanoparticles, ACS Sustainable Chemistry & Engineering, 7 (14), pp. 12464-12473. https://doi.org/10.1021/acssuschemeng.9b02035
Introduction
Before discussing the properties of graphene oxide with respect to CO2, the terms “adsorption” and “absorption” should be clarified.
As will be explained later, graphene oxide can adsorb and absorb CO2 in various nanomaterial configurations.
Absorption is often confused with “adsorption”.
This is the ability of a material to bind atoms, ions, or molecules of a gas, liquid, or solid.
In this case, the ability to attract CO2 to the surface of graphene oxide and keep it stuck, adhered, or fixed.
This attraction is similar to the “surface tension” that causes water droplets to coalesce into larger droplets when the distance between them is small enough.
Absorption is the property of a material to absorb, integrate, or combine with atoms, ions, or molecules of a gas, liquid, or solid.
In this case, graphene oxide has the ability to integrate CO2, although it should be noted that it does not do this on its own, as it requires nanocomposites and polymers from third parties.
Analyzed facts
The article analyzed here presents relevant information that would explain the role of graphene oxide in the fight against climate change.
The study by (Rodríguez-García, S.; Santiago, R.; López-Díaz, D.; Merchán, M.D.; Velázquez, M.M.; Fierro, J.L.G.; Palomar, J. 2019) demonstrates the “adsorptive” properties of graphene oxide in combination with Fe3O4 nanoparticles to reduce CO2 emissions into the atmosphere.
The combination of graphene oxide with Fe3O4 nanoparticles is directly related to the development of cancer drugs and DNA vaccines (Shah, MAA; He, N.; Li, Z.; Ali, Z.; Zhang, L. 2014), biocide fertilizers for agricultural use (Zhang, M .; Gao, B .; Chen, J .; Li, Y .; Creamer, AE; Chen, H. 2014), and 5G electromagnetic wave absorption tests (Ma, E . ; Li, J.; Zhao, N.; Liu, E.; He, C.; Shi, C. 2013), administration of vaccines with genetic reformulations using the CRISPR technique (Abbott, TR; Dhamdhere, G. ; Liu, Y. ; Lin, X.; Goudy, L.; Zeng, L.; Qi, LS 2020 | Ding, R.; Long, J.; Yuan, M.; Jin, Y.; Yang, H.; Chen, M.; Duan, G. 2021 | Teng, M.; Yao, Y.; Nair, V. Luo, J. 2021).
In other words, it is always the same highly versatile compound in all cases and applications.
Although graphene oxide has special properties that make it an ideal material for atmospheric filtration and air decontamination, it is paradoxical and contradictory at the same time.
It should not be forgotten that inhaled graphene oxide (in suspended particles) is harmful to health (Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Shao, L. 2016) and can cause significant damage, as already described in previous contributions, see graphene oxide in blood (Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. 2019), graphene oxide interaction with brain cells (Rauti, R .; Lozano, N .; León, V .; Scaini, D .; Musto, M .; Rago, I .; Ballerini, L. 2016), graphene oxide disrupts mitochondrial homeostasis (Xiaoli, F . ; Yaqing, Z .; Ruhui, L .; Xuan, L .; Aijie, C .; Yanli, Z .; Longquan, S. 2021), among other articles that can be retrieved via the query “graphene toxicity” .
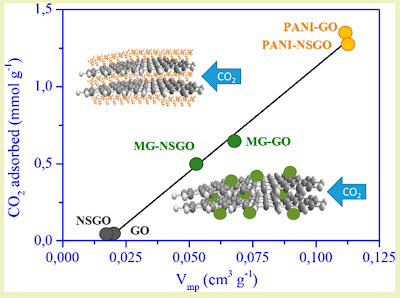
Returning to the analysis of the article, it states that graphene oxide nanocomposites with polyaniline (PANI) or Fe3O4 nanoparticles are capable of adsorbing and retaining CO2.
This particular capacity “increases linearly with the volume of the micropores“.
This detail is relevant because it matches the type of material used in ice nucleation, which also had to be porous to achieve better performance in nanocrystal formation (Liang, H.; Möhler, O.; Griffiths, S.; Zou, L. 2019).
The porous property is also appreciated in chemical fertilizers and, interestingly, in nanoparticles targeting RNA interference therapies in the brain (Joo, J.; Kwon, EJ; Kang, J.; Skalak, M.; Anglin, EJ; Mann, AP; Sailor, MJ 2016).
Indeed, porous graphene is used as an atmospheric nanofilter, as also reported by other authors (Blankenburg, S.; Bieri, M.; Fasel, R.; Müllen, K.; Pignedoli, C. A.; Passerone, D. 2010), in the form of 2D graphene oxide membranes adsorbing ammonia, CO2 and argon.
Reviewing the introduction of the article, other interesting statements can be observed.
Specifically, the justification for the research is summarized in the problem of global warming, which is “a serious problem for the planet.
The increasing concentration of greenhouse gases, especially CO₂, makes it necessary to develop processes for their elimination“.
In this sense, graphene oxide presents itself as an “efficient and economical solution” to mitigate the effects that this “pollutant” can cause.
Among the different options studied in the scientific literature (membrane adsorption and absorption or chemical adsorption in liquid amines), none stands out as having a good balance between performance, energy efficiency and impact.
However, graphene oxide in the form of hydrogels, aerogels, nanospheres and nanotubes seems to triple the CO2 capture capacity when functionalized with Fe3O4.
The experiment performed aims to simulate a realistic scenario of CO2 adsorption, specifically that produced by a combustion gas.
This suggests that one of the obvious applications of graphene oxide could be in the exhaust pipes of combustion engines or any other industrial combustion process.
In fact, the authors point out that in a more realistic scenario corresponding to a post-combustion gas (pCO2 = 0.15 bar and pN2 = 0.85 bar), the IAST CO2/N2 selectivity values obtained from nanocomposites prepared with graphene oxide need to be improved to ensure effective retention.
The IAST (Ideal Adsorbed Solution Theory) is determined by several factors, the most important of which are the atmospheric pressure expressed in “bar” (pressure unit), the atomic weight per gram in mmol/g of the graphene oxide catalyst, the temperature, the CO2 concentration and the adsorption time.
The researchers conclude that graphene oxide coated with the PANI polymer offers better adsorption results at operating temperatures, as well as recyclability properties that allow its modulable behavior for higher efficiency.
Other studies have also focused on CO2 “adsorption” with graphene oxide.
For example, the study by (Wu, X.; Zhao, B.; Wang, L.; Zhang, Z.; Zhang, H.; Zhao, X.; Guo, X. 2016) tested the behavior of PVDF (polyvinylidene fluoride) and graphene oxide at different concentrations to create membranes by observing CO2 adsorption under room temperature conditions.
It was concluded that increasing the percentage of graphene resulted in an increase in the adsorption capacity of the membrane.
This result was influenced by the porosity factor (82% in the experiment), which was also responsible for the crystallization or nucleation of PVDF, which caused a change in the shape of the membrane, with increased roughness and contact surface with graphene oxide, and consequently increased adsorption capacity.
Interestingly, the membrane did not lose CO2 even when wet due to the hydrophobic properties of PVDF.
(Irani, V.; Maleki, A.; Tavasoli, A., 2019) also studied CO2 adsorption with nanofluidized graphene oxide combined with MDEA, also known as “mehyldiethanolamine amine,” confirming the capabilities of the material.
For example, the addition of 0.2 percent graphene oxide to MDEA was shown to increase its CO2 adsorption capacity by more than 10 percent at different temperatures, with little increase in the weight of the mixture.
Final thoughts
Graphene oxide can be used to adsorb CO₂ from the atmosphere and contribute to the reduction of greenhouse gases.
In this sense, it would not be surprising if it is already being used for this purpose, since, according to (Pöschl U,. 2005), graphene oxide is found in the analysis of aerosols in the atmosphere, together with soot resulting from pyrolysis and incomplete combustion of jet aircraft, in a small but unquantified amount.
Bibliography
1.Abbott, TR; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Qi, LS (2020). Development of CRISPR as a prophylactic strategy to combat novel coronavirus and influenza. BioRxiv. https://doi.org/10.1101/2020.03.13.991307
2.Blankenburg, S.; Bieri, M.; Fasel, R.; Mullen, K.; Pignedoli, C.A.; Passerone, D. (2010). Porous graphene as an atmospheric nanofilter. Small, 6 (20), pp. 2266-2271. https://doi.org/10.1002/smll.201001126
3.Cabrera-Sanfelix, P. (2009). Adsorption and reactivity of CO2 on defective graphene sheets. The Journal of Physical Chemistry A, 113 (2), pp. 493-498. https://doi.org/10.1021/jp807087y
4.CN107585764A. Gang JU; Hai Yang; Cai-Min S.(2020). Porous oxidation graphene and preparation method thereof and porous oxidation graphene coated slow-release chemical fertilizer and preparation method thereof. https://patents.google.com/patent/CN107585764A/en
5.Ding, R.; Long J.; Yuan, M.; Jin, Y.; Yang, H.; Chen, M.; Duan, G. (2021). CRISPR/Cas System: A Potential Technology for the Prevention and Control of COVID-19 and Emerging Infectious Diseases. Frontiers in cellular and infection microbiology, 11. https://dx.doi.org/10.3389%2Ffcimb.2021.639108
6.Irani, V.; Maleki, A.; Tavasoli, A. (2019). CO2 absorption enhancement in graphene-oxide/MDEA nanofluid. Journal of environmental chemical engineering, 7 (1), 102782.
7.Joo, J.; Kwon, EJ; Kang, J.; Skalak, M.; Anglin, EJ; Mann, AP; Sailor, MJ (2016). Porous silicon–graphene oxide core–shell nanoparticles for targeted delivery of siRNA to the injured brain. Nanoscale Horizons, 1 (5), pp. 407-414. https://doi.org/10.1039/C6NH00082G
8.Liang, H.; Möhler, O.; Griffiths, S.; Zou, L. (2019). Enhanced Ice Nucleation and Growth by Porous Composite of RGO and Hydrophilic Silica Nanoparticles. The Journal of Physical Chemistry C, 124 (1), pp. 677-685. https://doi.org/10.1021/acs.jpcc.9b09749
9.Ma, E.; Li, J.; Zhao, N.; Liu, E.; Lui, C.; Shi, C. (2013). Preparation of reduced graphene oxide/Fe3O4 nanocomposite and its microwave electromagnetic properties. Materials Letters, 91, pp. 209-212. https://doi.org/10.1016/j.matlet.2012.09.097
10.Meconi, GM; Tomovska, R.; Zangi, R. (2019). Adsorption of CO2 gas on graphene–polymer composites. Journal of CO2 Utilization, 32, pp. 92-105. https://doi.org/10.1016/j.jcou.2019.03.005
11.Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Shao, L. (2016). Toxicity of graphene-family nanoparticles : a general review of the origins and mechanisms. Particle and Fibre Toxicology, 13 (1), pp. 1-24. https://doi.org/10.1186/s12989-016-0168-y
12.Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. (2019). Graphene oxide touches blood : in vivo interactions of bio-coronated 2D materials. Nanoscale Horizons, 4 (2), pp. 273-290. https://doi.org/10.1039/C8NH00318A
13.Poschl, U. (2005). Atmospheric aerosols: composition, transformation, climate and health effects. Angewandte Chemie International Edition, 44 (46), pp. 7520-7540. https://doi.org/10.1002/anie.200501122
14.Rauti, R.; Lozano, N.; Leon, V.; Scaini, D.; Musto, M.; Rago, I..; Ballerini, L. (2016). Graphene Oxide Nanosheets Reshape Synaptic Function in Cultured Brain Networks. ACS Nano, 10 (4), pp. 4459-4471. https://doi.org/10.1021/acsnano.6b00130
15.Shah, M.A.A.; He, N .; Li, Z.; Ali, Z.; Zhang, L. (2014). Nanoparticles for DNA Vaccine Delivery. Journal of Biomedical Nanotechnology, 10 (9), pp. 2332-2349. https://doi.org/10.1166/jbn.2014.1981
16.Teng, M.; Yao, Y.; Nair, V.; Luo, J. (2021). Latest Advances of Virology Research Using CRISPR/Cas9-Based Gene-Editing Technology and Its Application to Vaccine Development. Viruses, 13 (5), 779. https://doi.org/10.3390/v13050779
17.Wu, X.; Zhao, B.; Wang, L.; Zhang, Z.; Zhang, H.; Zhao, X.; Guo, X. (2016). Hydrophobic PVDF/graphene hybrid membrane for CO2 absorption in membrane contactor. Journal of Membrane Science, 520, pp. 120-129. https://doi.org/10.1016/j.memsci.2016.07.025
18.Xiaoli, F.; Yaqing, Z.; Ruhui, L.; Xuan, L.; Aijie, C.; Yanli, Z.; Longquan, S. (2021). Graphene oxide disrupted mitochondrial homeostasis through inducing intracellular redox deviation and autophagy-lysosomal network dysfunction in SH-SY5Y cells. Journal of Hazardous Materials,, 416, 126158. https://doi.org/10.1016/j.jhazmat.2021.126158
19.Zhang, M.; Gao, B.; Chen, J.; Li, Y.; Creamer, AE; Chen, H. (2014). Slow-release fertilizer encapsulated by graphene oxide films. Chemical Engineering Journal, 255, pp. 107-113. https://doi.org/10.1016/j.cej.2014.06.023