January 31, 2025 Graphene, Scientific alternative studies
In order to detect food contamination, it is important to identify which lactose-free dairy products have undergone these processes.
Lactose-free dairy products and graphene oxide. Possible Cause of Lactose Intolerance ?
Reference study
Trusek, A.; Dworakowska, D.; Czyzewska, K. (2020). 3D enzymatic preparations with graphene oxide flakes and hydrogel to obtain lactose-free products. Food and Bioproducts Processing, 121, pp. 224-229. https://doi.org/10.1016/j.fbp.2020.03.002
Introduction
Lactose intolerance is a problem caused by the inability of the small intestine to produce lactase, the enzyme that allows lactose to be broken down into glucose and galactose.
This can cause diarrhea, gas, bloating, and indigestion after ingesting dairy products.
Lactose-free dairy products are generally designed to make digestion easier for people who are lactose intolerant, thus avoiding the aforementioned conditions and problems.
In this post you will find that many methods used to produce lactose-free foods involve the use of graphene oxide.
Analyzed facts
The research of (Trusek, A.; Dworakowska, D.; Czyzewska, K. ,2020) shows that GO graphene oxide can be used as a vehicle in enzyme immobilization, allowing to inhibit the enzyme responsible for lactose production in fermentation processes of dairy products.
For this reason, “the article develops a method of chemical activation prior to immobilization of enzyme molecules.
This property of graphene oxide allows the immobilization of the β-galactosidase enzyme after GO activation with divinylsulfone“.
β-Galactosidase (or beta-galactosidase) is the enzyme used to produce lactose-free products.
β-Galactosidase is also responsible for the fermentation of lactose sugars that allow the production of cheese, yogurt, and other dairy products; in other words, it is an enzyme that catalyzes the hydrolysis of galactosides to monosaccharides.
As for “divinylsulfone“, it is a chemical compound with the molecular structure “C4H6O2S” that can be derived from mustard gas (Grant, WM; Thomas, CC 1987), which is considered a hazardous product because of its toxic, corrosive and irritant properties.
Divinylsulfone has been used in other cases for the preparation of porous hydrogels (Sannino, A.; Madaghiele, M.; Conversano, F.; Mele, G.; Maffezzoli, A.; Netti, PA; Nicolais, L. 2004) and for the preparation of drugs and encapsulating agents (Morales-Sanfrutos, J. ; Lopez-Jaramillo, FJ; Elremaily, MA; Hernández-Mateo, F.; Santoyo-Gonzalez, F. 2015) or for activating the properties of other compounds such as chitosan (Pinheiro, BB; Rios, NS; Aguado, ER; Fernandez-Lafuente, R.; Freire, TM; Fechine, PB; Goncalves, LR 2019).
The authors state that the process of lactose separation by this method is efficient and fast, allowing to obtain a low concentration of lactose in the effluent stream at a very low temperature, 6°C, which corresponds to the storage conditions of refrigerated milk.
To solve these problems, the authors developed two basic approaches : first, enzymatic separation, solved by combining GO flakes with divinyl sulfone to separate and isolate the β-galactosidase enzyme, and second, the development of a less expensive production method.
The authors summarize the problem as follows : “The main disadvantage of flake graphene vehicles is the difficulty in separating them from solution due to the size and density of the particles.
This drawback creates problems during substrate preparation and application.
In previous work, a GO flake separation method based on ultrafast centrifugation was developed (Trusek, A. 2019).
This method was efficient but expensive, especially for large-scale applications.
To overcome this problem, 3D preparations based on encapsulation of GO flakes were proposed.
The new method combines applications of hydrogels and GO“.
Although the method allows the elimination of lactose at low cost, the authors do not explain the elimination process.
In fact, they hypothesize the release of graphene flakes into the solution in combination with the lactose, as reported in the next paragraph : “The encapsulation of graphene in the hydrogel flakes allowed the preparations to be easily obtained and packed in the chemical distillation reactor column.
There was no hydraulic resistance during the flow of the substrate through the column.
In addition, the alginate capsules did not break, thus preventing the release of the graphene flakes into the solution“.
This raises many questions and unresolved issues in the article, given that the food product is in direct contact with the solution containing graphene flakes.
Among the conclusions, it should be noted that “the procedure developed for the preparation of 3D vectors can be used for any enzyme“.
This means that graphene oxide, along with other components, can be used to inhibit or immobilize all types of enzymes.
This assertion is confirmed by many other researchers, as can be seen in (Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S., 2010), who consider graphene oxide as “the matrix for enzyme immobilization“; in fact, they state that “immobilization of the enzyme on GO sheets could be easily achieved without the use of cross-linking reagents or further surface modification“.
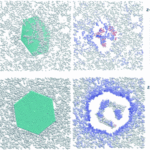
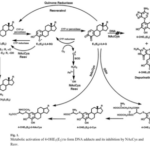
This is due to the adsorption capacity of graphene, which causes the inhibition of the enzyme, as shown in the graph of the immobilization reaction rate distribution, see Figure 1.
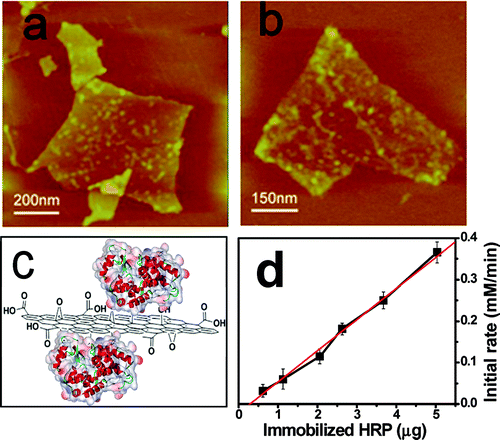
Since graphene oxide has special properties for immobilizing enzymes, this technique has been studied for immobilizing hydrolases, a family of digestive enzymes, including lactase, which is listed in the EC3 classification under the code “3.2.1.108” of the glucosidase subfamily.
According to Husain (2016), it is possible to immobilize hydrolases with magnetic nanoparticles, including graphene oxide in combination with magnetite Fe3O4.
Therefore, it is possible that GO graphene oxide alone or in combination with other components in the small intestine can inhibit lactase, which is responsible for lactose digestion, causing lactose intolerance.
This immobilization effect is also confirmed by (Chen, L.; Wei, B.; Zhang, X.; Li, C., 2013), which interestingly uses graphene and Fe₂O₃ aerogel (or iron trioxide) with higher saturation magnetization (23-54 emu/g-¹).
Emu/g (magnetic unit to define the ratio of magnetization per mass) is a unit of measurement used in magnetism.
Another similar study is by Jiang et al. (2012) in which “magnetic graphene oxide nanocomposites modified with Fe3O4 nanoparticles succeeded in immobilizing trypsin“.
Trypsin is an essential digestive enzyme that is produced in the pancreas and secreted into the duodenum to hydrolyze peptides that promote the absorption of proteins in food.
Other studies
Other studies confirm the role of graphene oxide in the production of lactose-free dairy products.
An example is the study by (Morelos-Gomez, A.; Terashima, S.; Yamanaka, A.; Cruz-Silva, R.; Ortiz-Medina, J.; Sánchez-Salas, R.; Endo, M., 2021) who developed graphene oxide membranes for lactose-free milk.
The membranes are capable of filtering lactose with a permeation capacity of more than 2.5 kg per m2 per day.
The authors state that “molecular dynamics (MD) simulations show that lactose has mainly weak van der Waals interactions with the GO layers, allowing lactose to diffuse through the nanocannals of the GO membranes while leaving fat and protein in the milk“.
These filtration properties are similar to those found in wastewater filtration (Fathizadeh, M. ; Xu, WL ; Zhou, F. ; Yoon, Y. ; Yu, M. 2017 | Wang, J. ; Zhang, P .; Liang, B .; Liu, Y . Xu, T. ; Wang, L. ; Pan, K. 2016), including uranium (Hu, X. ; Wang, Y. ; Yang, J.O. ; Li, Y. ; Wu, P. ; Zhang, H. ; Liu, Z. 2020 | Li, Z. ; Chen, F. ; Yuan, L. ; Liu, Y. ; Zhao, Y. ; Chai, Z. ; Shi, W. 2012).
However, as in the other studies, no residues or traces of graphene in milk and other dairy products are analyzed, which may cause intoxication or poisoning of consumers, with all the side effects and damages caused by graphene oxide.
Worthy of special attention is the work of (De-Brito, AR; de-Carvalho-Tavares, IM; De-Carvalho, MS; De-Oliveira, AJ; Salay, LC; Santos AS; Franco, M., 2020) who study the interaction of “lactase” in a matrix of CNT carbon nanotubes, which are cylindrical graphene nanosheets.
The researchers show that “the enzyme lactase is absorbed in the tubular region of carbon nanotubes.
Fluorescence spectroscopic analysis showed that the fluorescence emission of lactase was mainly due to the tryptophan residue (TRP) and that this fluorescence was reduced in the presence of CNTs, demonstrating the interaction between these components“.
This statement strengthens the argument that graphene oxide could inhibit or immobilize many of the digestive enzymes, causing lactose intolerance problems and other possible contraindications that have not yet been discovered.

Another example of enzyme immobilization is reported by Zhou, L. et al. (2012), in which “glucose oxidase” is inhibited with graphene oxide.
Glucose oxidase is the enzyme that helps break down sugars to promote their metabolism.
Obviously, when glucose oxidase is immobilized, metabolic dysfunction could occur.
Although the study was aimed at the development of bioelectrodes and biosensors, it shows that graphene oxide can interfere with the metabolic function of the body, as suggested and stated previously by (Papi, M. ; Lauriola, MC; Palmieri, V. ; Ciasca, G. ; Maulucci, G. ; De-Spirito, M. 2015 | Volkov, Y. ; McIntyre, J.. ; Prina-Mello, A. 2017; Zhang, Y.; Qin, L.; Sun, J.; Chen, L.; Jia, L.; Zhao, J.; Sang, W. 2020; Jastrzębska, AM, Kurtycz, P. and Olszyna, AR 2012; Singh, Z. 2016 | Jarosz, A.; Skoda, M.; Dudek, I.; Szukiewicz, D. 2016 | Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, AA; Tretiach, M.; Syrgiannis, Z. 2016).
Final reflections
It has been shown that lactose-free dairy products could be the result of the application of graphene oxide filtration techniques aimed at eliminating lactose.
However, none of the studies consulted analyzed the possible residues of graphene oxide in food products, nor the toxicity and adverse effects that this compound may have on the human body.
It is essential to identify which lactose-free dairy products have undergone these processes in order to detect food contamination.
This requires laboratory analysis.
The researchers also confirm the ability of graphene oxide in nanoparticles, alone or in combination with other magnetic elements such as Fe2O3 and Fe3O4, to inhibit or immobilize all types of enzymes present in the small intestine.
The incidence of enzymes in other organs has not yet been analyzed, but scientific literature confirms that the involvement could be more extensive, as graphene oxide affects cell metabolism (Jarosz, A.; Skoda, M.; Dudek, I.; Szukiewicz, D., 2016).
In this article, oxidative stress and disruption of mitochondrial homeostasis are explained.
Hypothesis
People who have received the so-called “vaccine” may develop lactose intolerance and problems related to enzyme immobilization because graphene oxide may interfere with its proper functioning.
It could also be the case that people with lactose intolerance have high concentrations of graphene oxide or metal nanoparticles in the small intestine.
On the other hand, graphene oxide could interfere with the normal functioning of trypsin as it can inhibit enzymes, which would explain indigestion, nausea, reflux, abdominal pain and even diarrhea.
Bibliography
1.Chen, L.; Wei, B.; Zhang, X.; Li, C. (2013). Bifunctional graphene/γ‐Fe2O3 hybrid aerogels with double nanocrystalline networks for enzyme immobilization. Piccolo, 9 (13), pp. 2331-2340. https://doi.org/10.1002/smll.201202923
2.De-Brito, AR; de-Carvalho-Tavares, IM; de-Carvalho, MS; de-Oliveira, AJ; Salay, LC; Santos, AS; Franco, M. (2020). Study of the interaction of the lactase enzyme immobilized in a carbon nanotube matrix for the development of the chemically modified carbon paste electrode. Surfaces and interface, 20, 100592. https://doi.org/10.1016/j.surfin.2020.100592
3.Fathizadeh, M.; Xu, WL; Zhou, F.; Yoon, Y.; Yu, M. (2017). Graphene Oxide: A Novel 2-Dimensional Material in Membrane Separation for Water Purification. Advanced Materials Interfaces, 4 (5), 1600918. https://doi.org/10.1002/admi.201600918
4.Grant, WM; Tommaso, CC (1987). Toxicology of the eye. Journal of Toxicology : Cutaneous and Ocular Toxicolog, 6 (2), pp. 155-156. https://doi.org/10.3109/15569528709052171
5.Husayn, Q. (2016). Magnetic nanoparticles as a tool for the immobilization/stabilization of hydrolases and their applications : An overview. Biointerface Research in Applied Chemistry, 6 (6). https://www.researchgate.net/publication/311842151_Magnetic_nanoparticles_as_a_tool_for_the_immobilizationstabilization_of_hydrolases_and_loro_applicazioni_Una_panoramica
6.Hu, X.; Wang, Y.; Yang, JO; Li, Y.; Wu, P.; Zhang, H.; Liu, Z. (2020). Synthesis of graphene oxide nanoribbons/chitosan composite membranes for the removal of uranium from aqueous solutions. Frontiers of Chemical Science and Engineering, 14 (6), pp. 1029-1038. https://doi.org/10.1007/s11705-019-1898-9
7.Jastrzębska, AM, Kurtycz, P. e Olszyna, AR (2012). Recent advances in graphene family materials toxicity investigations . Journal of Nanoparticle Research volume, 14 (12), pp. 1-21. https://doi.org/10.1007/s11051-012-1320-8
8.Jarosz, A.; Skoda, M.; Dudek, io.; Szukiewicz, D. (2016). Oxidative stress and mitochondrial activation as the main mechanisms underlying graphene toxicity against human cancer cells. Oxidative medicine and cellular longevity . https://doi.org/10.1155/2016/5851035
9.Jiang, B.; Yang, K.; Zhao, Q.; Wu, Q.; Liang, Z.; Zhang, L.; Zhang, Y. (2012). Hydrophilic immobilized trypsin reactor with magnetic graphene oxide as support for high efficient proteome digestion. Journal of chromatography A, 1254, pp. 8-13. https://doi.org/10.1016/j.chroma.2012.07.030
10.Li, Z.; Chen, F.; Yuan, L.; Liu, Y.; Zhao, Y.; Chai, Z.; Shi, W. (2012).Uranium (VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chemical engineering journal, 210, pp. 539-546. https://doi.org/10.1016/j.cej.2012.09.030
11.Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, AA; Tretiach, M.; Syrgiannis, Z. (2016). Ecotoxicological effects of graphene-based materials. 2D Materials, 4 (1), 012001. https://doi.org/10.1088/2053-1583/4/1/012001
12.Morales-Sanfrutos, J.; Lopez-Jaramillo, FJ; Elremaily, MA; Hernández-Mateo, F.; Santoyo-González, F. (2015). Divinyl Sulfone Cross-Linked Cyclodextrin-Based Polymeric Materials : Synthesis and Applications as Sorbents and Encapsulating Agents. Molecules, 20 (3), pp. 3565-3581. https://doi.org/10.3390/molecules20033565
13.Morelos-Gomez, A.; Terashima, S.; Yamanaka, A.; Cruz-Silva, R.; Ortiz-Medina, J.; Sánchez-Salas, R.; Endo, M. (2021). Graphene oxide membranes for lactose-free milk. Carbon, 181, pp. 118-129. https://doi.org/10.1016/j.carbon.2021.05.005
14.Papi, M.; Lauriola, MC; Palmieri, V.; Ciasca, G.; Maulucci, G.; De-Spirito, M. (2015). Plasma protein corona reduces the haemolytic activity of graphene oxide nano and micro flakes. RSC Advances, 5 (99), pp. 81638-81641. https://doi.org/10.1039/C5RA15083C
15.Pinheiro, BB; Rios, NS; Aguado, ER; Fernandez-Lafuente, R.; Freire, TM; Fechine, PB; Goncalves, LR (2019). Chitosan activated with divinyl sulfone : a new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. International journal of biological macromolecules, 130, pp. 798-809. https://doi.org/10.1016/j.ijbiomac.2019.02.145
16.Sannino, A.; Madaghiele, M.; Conversano, F.; Mele, G.; Maffezzoli, A.; Netti, PA; Nicolais, L. (2004). idCellulose Derivative−Hyaluronic Acid-Based Microporous Hydrogels Cross-Linked through Divinyl Sulfone (DVS) To Modulate Equilibrium Sorption Capacity and Network Stability. Biomacromoléculas, 5 (1), pp. 92-96. https://doi.org/10.1021/bm0341881
17.Singh, Z. (2016). Toxicity of graphene and its nanocomposites to human cell lines-the present scenario. International Journal of Biomedical and Clinical Sciences 1 (1), pp. 24-29. http://files.aiscience.org/journal/article/pdf/70570032.pdf
18.Trusek, A. (2019). Graphene oxide flake activation via divinylsulfone–a procedure for efficient β-galactosidase immobilization. Polish Journal of Chemical Technology, 21 (1). http://dx.doi.org/10.2478/pjct-2019-0006
19.Trusek, A., Dworakowska, D. e Czyzewska, K. (2020).3D enzymatic preparations with graphene oxide flakes and hydrogel to obtain lactose-free products. Food and Bioproducts Processing, 121, pp. 224-229. https://doi.org/10.1016/j.fbp.2020.03.002
20.Volkov, Y.; McIntyre, J.; Prina-Mello, A. (2017). Graphene toxicity as a double-edged sword of risks and exploitable opportunities: a critical analysis of the most recent trends and developments. 2D Materials, 4 (2), 022001. https://doi.org/10.1088/2053-1583/aa5476
21.Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Pan, K. (2016). Graphene Oxide as an Effective Barrier on a Porous Nanofibrous Membrane for Water Treatment. ACS applied materials & interfaces, 8 (9), pp. 6211-6218. https://doi.org/10.1021/acsami.5b12723
22.Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. (2010). Graphene oxide as a matrix for enzyme immobilization. Langmuir, 26 (9), pp. 6083-6085. https://doi.org/10.1021/la904014z
23.Zhang, Y.; Qin, L.; Sun, J.; Chen, L.; Jia, L.; Zhao, J.; Sang, W. (2020). Metabolite changes associated with earthworms (Eisenia fetida) graphene exposure revealed by matrix-assisted laser desorption/ionization mass spectrometry imaging. Ecotoxicology and Environmental Safety, 205, 111102. https://doi.org/10.1016/j.ecoenv.2020.111102
24.Zhou, L.; Jiang, Y.; Gao, J.; Zhao, X.; Ma, L..; Zhou, Q. (2012). Oriented immobilization of glucose oxidase on graphene oxide. Biochemical engineering journal, 69, pp. 28-31. https://doi.org/10.1016/j.bej.2012.07.025